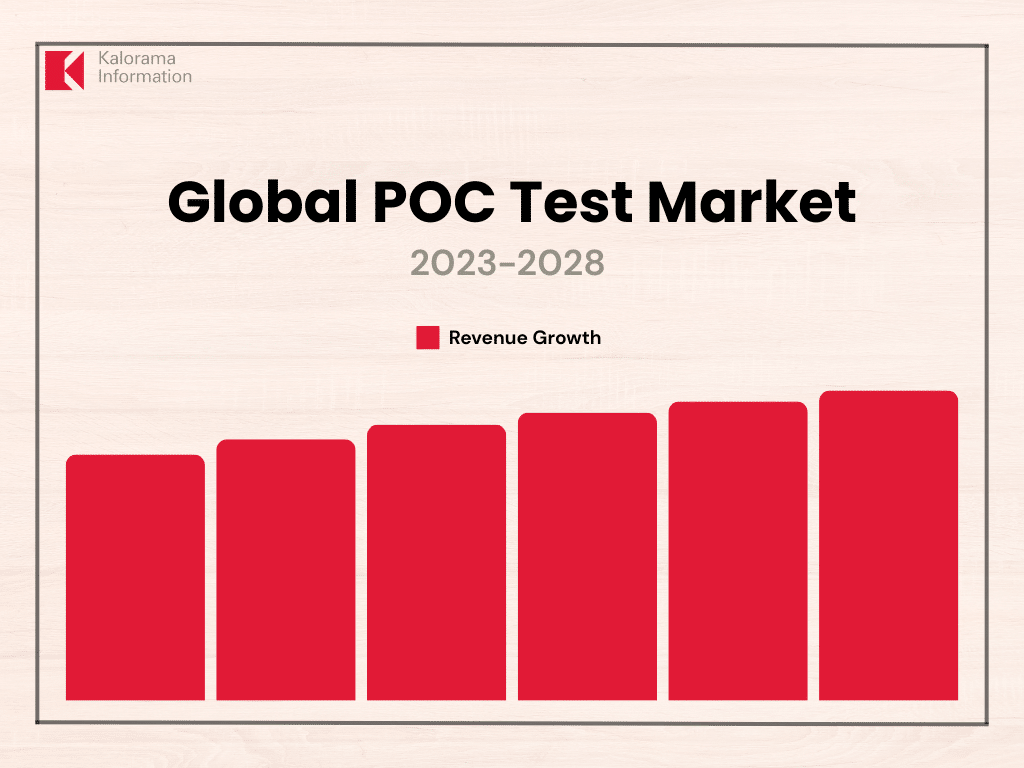
In 2023, point-of-care (POC) diagnostic testing market sales reached $36.8 billion, fueled by both professional and self-testing, according to Kalorama Information’s April 2024 report The Worldwide Market for Point-of-Care (POC) Diagnostic Tests, 11th Edition. The POC diagnostic testing market continues to expand thanks to emerging technologies and increasing demand that will drive growth into the foreseeable future.
Post-Pandemic Growth and Trends
Prevalence of chronic and infectious disease worldwide has helped to increase the need for faster turnaround times of POC diagnostics. Early detection of disease and treatment is a focus for all areas of in vitro diagnostics (IVD) and POC plays an integral role in the process. As a result, the point-of-care industry is one of the most consistently growing segments of the IVD industry.
In 2023, the POC market resumed its pre-pandemic growth trajectory across all segments. While COVID-19 testing still contributes to revenues, its impact has diminished. Other diagnostic segments are thriving even as COVID testing falters, and this positive trend is expected to continue, with the market projected to grow by nearly 5% annually through 2028.
Several factors are shaping the future of the POC diagnostic testing market:
- Wearables and Mobile Health Technologies: These innovations are enhancing patient-centered healthcare by enabling real-time monitoring and diagnostics.
- Telehealth: The integration of telehealth with POC testing allows for immediate diagnostic feedback and remote patient management.
- Breath Test Advances: New non-invasive diagnostic methods are being developed, offering a promising future for POC testing.
- Regulatory and Reimbursement Challenges: Navigating these hurdles is critical for market players to ensure widespread adoption of new POC technologies.
- Emerging Technologies: Advances in biosensors, microfluidics, and AI are driving the development of more sophisticated and accurate POC tests.
Expanding the POC Testing Menu
POC testing has expanded beyond traditional tests such as blood glucose, electrolytes, and infectious diseases. Recent additions include tests for HbA1c, BNP, D-Dimer, and CRP, with more innovations on the horizon. These advancements have been instrumental in migrating POC testing from hospitals to a variety of settings, including workplaces, homes, and convenience clinics.
The Role of POC in Personalized Medicine
POC diagnostics are integral to the shift towards personalized medicine, where rapid, accurate tests facilitate early disease detection and tailored treatment. The demand for such diagnostics has spurred significant technological innovations, particularly in molecular POC tests for respiratory and sexually transmitted infections.
Market Dynamics and Future Prospects
- Decentralized Testing: The movement of POC testing outside the central laboratory is growing, driven by the availability of portable and handheld instruments.
- Diagnostic Connectivity: New POC devices now offer lab-quality results that can be automatically transferred to electronic medical records or remote caregiver services, enhancing patient care.
- Molecular Diagnostics: The adoption of molecular POC tests in physician offices is increasing, providing timely and precise diagnostic information.
- Cost Considerations: While pricing strategies have varied, the adoption of POC tests is increasingly based on performance, patient outcomes, and cost-benefit analysis rather than price alone.
The Future of POC Testing
The POC diagnostic market is poised for continued growth, driven by innovations in technology and a growing need for rapid, accurate diagnostics. Industry stakeholders must stay informed about regulatory changes and reimbursement policies to capitalize on emerging opportunities.
As we look to the future, the POC industry will play a crucial role in the transition to predictive, personalized, and preemptive medicine, ensuring that patients receive the right diagnosis and treatment at the right time, wherever they are.
For more information, consider purchasing The Worldwide Market for Point-of-Care (POC) Diagnostic Tests, 11th Edition, a report built on Kalorama’s two decades of observing and analyzing the POC industry.